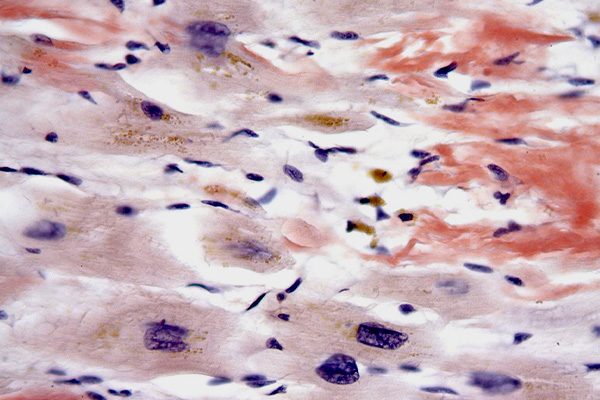
Llewellyn's Sirius Red
for Amyloid
Materials
| Material | Amount | |
|---|---|---|
| Sirius red F3B | 0.5 | g |
| Distilled water | 50 | mL |
| Ethanol, absolute | 50 | mL |
Staining Solution Preparation
- Dissolve the dye into the water, add ethanol and mix well.
- Add 1 mL of 1% sodium hydroxide. Then, while strong backlighting and swirling, add drops of 20% sodium chloride until a fine haze is detected. Usually about 2 mL is adequate. Adding more than 4 mL causes excessive precipitation. The solution is reasonably stable for several months, but slowly deteriorates. Extend the staining time to compensate. When it requires more than 2 hours to adequately stain, prepare a new solution.
Tissue Sample
5µ paraffin sections of neutral buffered formalin fixed tissue are suitable. Other fixatives are likely to be satisfactory.
Protocol
- Bring sections to water via xylene and ethanol.
- Stain nuclei with a progressive alum hematoxylin for a few minutes.
- Rinse with tap water.
- Rinse with ethanol.
- Place into alkaline sirius red for 1 – 2 hours.
- Rinse well with tap water.
- Dehydrate with absolute ethanol.
- Clear with xylene and mount with a resinous medium.
Expected Results
- Amyloid – red
- Eosinophil and Paneth cell granules – red
- Nuclei – blue
- Background – colorless


Notes
- Amyloid displays deep green birefringence when viewed with crossed polarisers, one above and one below the section.
- Eosinophils and Paneth cell granules are also demonstrated. If used for this purpose the sodium chloride may be ommitted.
- This method uses sirius red F3B. The dye Sirius red 4B is not suitable.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Llewellyn, B.D., (1970)
An improved sirius red method for amyloid.
Journal of Medical Laboratory Technology, v 23, 308