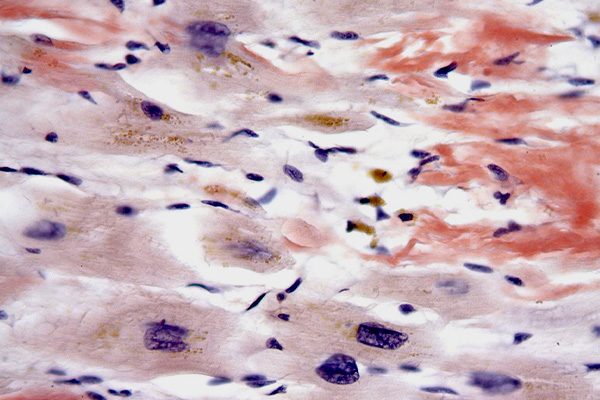
Wolman's Standard Toluidine Blue(STB)
for Amyloid
Materials
| Material | Amount | |
|---|---|---|
| Toluidine blue | 1 | g |
| Distilled water | 50 | mL |
| Iso-propanol, absolute | 50 | mL |
Tissue Sample
5µ paraffin sections of neutral buffered formalin fixed tissue are suitable. Other fixatives are likely to be satisfactory.
Protocol
- Bring sections to water via xylene and ethanol.
- Place in the toluidine blue solution at 37°C for 30 minutes.
- Blot carefully.
- Place into absolute iso-propanol for one minute.
- Blot carefully.
- Clear with xylene and coverslip using Canada balsam.
- Examine microscopically using crossed polarizing filters.
Expected Results
- Amyloid – orange-red to red birefringence.
- Orthochromatic tissue – blue-white birefringence
- metachromatic tissue – yellow-green birefringence
Notes
- Wolman strongly recommended this procedure, considering it to be highly selective for amyloid.
- The birefringence is independent of section thickness and the quality of the microscope optics.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Wolman, M. (1971).
Amyloid, its nature and molecular structure: comparison of a new toluidine blue polarized light method with traditional procedures.
Laboratory Investigation, v. 25: p. 104-110.