Virchow coined the name amyloid for a material he observed in tissues. This term means starch like, and is a reference to the earliest methods used to demonstrate its presence. The classical method to detect starch is to treat it with an iodine solution, when it turns blue. Amyloid initially stains deep brown with an iodine solution, but this turns blue after treatment with concentrated sulphuric acid. This is actually closer to cellulose than to starch, but at the time referencing an animal material as being the same as a plant material was not acceptable, so it was named starch like rather than cellulose like (celluloid, now used for another material derived from cellulose).
Introduction
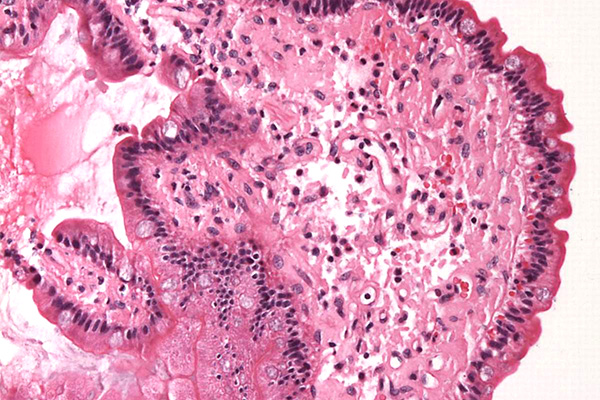
Amyloid is an acidophil, hyaline (glassy) material; that is, it is stained by acid dyes and is pale pink in an H & E, although its appearance is not sufficiently distinct to definitively identify it, and special stains are required. Most of these depend on a characteristic of amyloid that causes dyes to attach in a regular molecular pattern, forming a pseudocrystal. This word means false crystal, and describes the optical properties of amyloid that are similar to those shown by some crystals, and which are attributable to their very regular molecular structure.
Amyloid is largely protein, but it may contain other materials. For that reason sometimes it will be stained by methods for carbohydrates (alcian blue, PAS). In fact, this emphasizes the point that amyloid is invariably identified in light microscopy according to its staining characteristics rather than its morphology. In electron microscopy it has a particular fibrillar ultrastructure which identifies it specifically, but this cannot be seen with a light microscope.
What Causes Amyloid?
It used to be thought that amyloid was caused by some plasma constituent extruding into the tissues, much like fibrin is formed from fibrinogen in the plasma. Then it was considered to be the results of the reactions between antigens and antibodies which, for some reason, were deposited in the tissues rather than being removed. From the links in the chart below, it appears there may be several causes for the deposition of abnormal proteins as amyloid, including the presence of particular genes. It is not a single substance and there is no single cause for it, but a common thread is that the protein in amyloid is a β-pleated sheet instead of the more usual α-helix. The subject may be investigated by internet searches on the subjects of amyloid, amyloidosis, and the individual amyloid types in the list below. The reader is encouraged to do so as it is a large and quite complicated subject. There is a modern overview at Medscape.
Amyloid Identification Criteria
Wolman gave ten criteria for determining whether a material should be identified as amyloid or something else, adding that his Standard Toluidine Blue (STB) staining method should also be positive.
- It is acidophil in an H&E stain.
- It does not stain as collagen with Van Gieson’s picro-fuchsin or trichromes.
- It is weakly basophil with alcian blue and toluidine blue.
- It stains metachromatically with crystal violet and toluidine blue.
- It stains with congo red and sirius red F3B.
- Congo red and sirius red stained amyloid show green birefringence.
- It can be stained with trypan blue.
- It is positive with the p-dimethylaminobenzaldehyde (DMAB) technique for tryptophan.
- It exhibits fluorescence when stained with thioflavine T and thioflavine S.
- It has a particular fibrillar ultrastructure.
Electron Microscope Appearance
As previously noted, the only definitive way to identify amyloid is by its appearance with an electron microscope. Cohen & Calkins and Gueft & Ghidoni described it as extracellular fibers, often in small bundles. Each fibril is 75 Angstroms wide and composed of three linear bands, each 25 Angstroms wide. The fibrils are beaded at 100 Angstrom intervals.
Staining Methods
Metal Impregnation for Amyloid
Both silver and gold have been employed for the metallic impregnation of amyloid. Either will selectively demonstrate the material, but have not been proven to be specific. King’s silver method is quite straightforward but requires free floating frozen sections. Staining involves simply treating formalin fixed, free floating sections with an ammoniacal silver solution until stained. No chemical reduction is used. Lynch and Inwood’s gold impregnation depends on the affinity of amyloid for iodine. Sections are sequentially treated with iodine then gold. Finally, the product is oxidized with hydrogen peroxide.
These methods are not popular. Partly this is due to the expense of silver and gold, but also due to the simplicity and convenience of other dye staining methods.
Variability of Staining
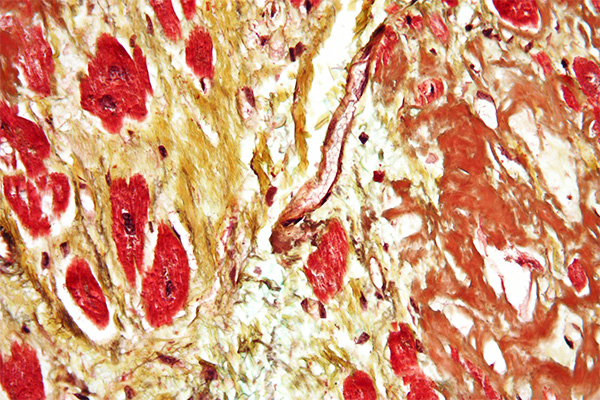
There is some disagreement as to which staining methods are the most appropriate for determining whether a material is amyloid or something else. Drury and Wallington describe amyloid as being eosinophil, moderately PAS positive, variable with a Van Gieson and blue or green with a Masson type trichrome, disagreeing with Wolman, who said that amyloid is not stained like collagen (i.e. blue or green) in a trichrome. Others require different staining results for a positive identification, such as a positive staining with congo red in conjunction with the DMAB nitrite test for tryptophane, or crystal violet metachromasia in conjunction with green birefringence after congo red staining.
These differences of opinion as to what constitutes definitive staining results for amyloid are rooted in the inherent variability of the material itself. Many examples of amyloid will meet the stated criteria, but it is not by any means unusual for particular examples to stain according to a different pattern. For example, during the author’s career he has had at least three examples in which the staining results caused confusion. In one, the congo red stain (Highman’s) was positive, but sirius red (Llewellyn’s) failed to stain it. In another the staining was reversed, with the congo red failing to stain with a positive sirius red. In the third, both congo red and sirius red were negative, as was crystal violet metachromasia, but Vassar and Culling’s thioflavine T was weakly positive. The material was later confirmed to be amyloid by electron microscopy.
The point is that amyloid is a variable material and the staining may be variable as well. Particular examples of amyloid may stain with a particular method or not, and failure to stain may not mean that a particular material is definitively not amyloid. In practice, each laboratory should make available several staining methods, so that negative results which were expected to demonstrate amyloid can be checked with alternate procedures, or, as a last resort, sent for electron microscopy. Either congo red or sirius red is suggested as the initial staining method, followed by whichever of those two was not used initially, along with thioflavine T. In addition, crystal violet metachromasia, standard toluidine blue, alcian blue and the DMAB nitrite test should also be available for those occasions when these initial methods are unexpectedly unsuccessful.
Tryptophan
Both amyloid and fibrin have elevated levels of tryptophan, so both materials will be stained by the DMAB nitrite test. This means that the technique cannot be used as a primary staining method for amyloid, since positively stained hyaline could be either amyloid or fibrin, but it can be used as a confirmatory stain when other staining methods are positive.
Carbohydrate
Amyloid often stains with alcian blue, thus indicating the presence of acid mucopolysaccharide. Both sulphated and non-sulfated types have been suggested, and it is quite possible that both may be present. Although amyloid is metachromatic with toluidine blue and crystal violet, the metachromasia may be dependent on a feature of the material other than the mucopolysaccharide content (i.e. its structure), and the metachromasia is therefore an unreliable indicator of sulfated acid mucopolysaccharide content in this particular case. On the other hand, the assumption sometimes made that alcian blue staining is merely a reflection of the mucopolysaccharides in the ground substance is unlikely, since amyloid is stained more darkly than the ground substance, indicating that it has a higher content.
Lendrum, Slidders and Fraser noted that alcian blue staining is more intense in fresh deposits of amyloid, suggesting that the alcian blue staining material may be being blocked or removed as the amyloid ages. In the same paper, these authors also comment on the variably of amyloid staining with a PAS. They observed that amyloid is initially PAS negative, then becomes positive, then once again becomes negative as the deposit ages. They used this progression and the variability with alcian blue to support their contention that amyloid undergoes changes in chemical composition and hence in staining as the deposit ages. However, this variability of alcian blue and PAS staining once again emphasizes the inherent difficulty in recommending a single staining method to identify the material.
β-pleated Sheet
The selectivity of many methods for amyloid appears to depend on two things: hydrogen bonding and the way that its amino acids fold.
In most proteins, amino acids are folded in a helical manner, rotating around a central point, then deforming the coil as various exposed groups come in contact and influence each other, whether by ionic or non-ionic forces. Amyloid has a different structure, called a beta pleated sheet (β-pleated sheet), which is composed of regular folds like a concertina. This is similar to a sheet of paper folded longitudinally in alternate directions making a zig-zag pattern when viewed on end (see to the right). The dye is often positioned within the creases of the folds, producing a regular alignment of dye molecules (shown in red), much like a crystal would have. This arrangement is referred to as a pseudocrystal, because it causes amyloid to exhibit some of the optical properties of a crystal, such as dichroism and dichroic birefringence. It may also explain the metachromasia with crystal violet.
Classifying Amyloid
The names of the types of amyloid usually start with the letter “A”. The following list of the most common types was made by blending the two lists in the Wikipedia articles referenced below and adding some links. The list is not exhaustive.
| Abbreviation | Amyloid type / gene | Description |
|---|---|---|
| AL | Amyloid light chain | AL amyloidosis / multiple myeloma. Contains immunoglobulin light-chains (λ,κ) derived from plasma cells. |
| AA | SAA | AA amyloidosis, rheumatoid arthritis |
| Aβ | β Amyloid / APP | Found in Alzheimer disease brain lesions. |
| Aβ2M | β2 Microglobulin | Hemodialysis-associated amyloidosis. |
| ATTR | Transthyretin / TTR | Familial amyloid polyneuropathies, senile systemic amyloidosis, leptomeningeal amyloidosis. |
| AIAPP | Amylin / IAPP | Pancreatic amyloid in type 2 diabetes. |
| AGel | Gelsolin, GSN | Finnish type amyloidosis. |
| AApoA1 | APOA1 | Familial visceral amyloidosis, atherosclerosis. |
| AFib | FGA | Familial visceral amyloidosis. |
| ALys | LYZ | Familial visceral amyloidosis. |
| ABri | ITM2B | Cerebral amyloid angiopathy, British-type |
| ADan | ITM2B | Cerebral amyloid angiopathy, Danish-type. |
| ACys | CST3 | Cerebral amyloid angiopathy, Icelandic-type. |
| APro | Prolactin | Prolactinoma. |
| AKer | Keratoepithelin | Lattice corneal dystrophy. |
| ACal | Calcitonin | Medullary carcinoma of the thyroid. |
| AANF | Atrial natriuretic factor | Senile amyloid of atria of heart, cardiac arrhythmias. |
| AMed | Medin | Aortic medial amyloid. |
| — | OSMR | Primary cutaneous amyloidosis. |
| — | Alpha-synuclein | Parkinson’s disease. |
| — | Huntingtin | Huntington’s Disease. |
| APrP | Prion protein | Deposits in prion diseases may resemble amyloid, i.e. in Creutzfeldt-Jakob disease, Kuru, BSE, and scrapie. |
From a histological perspective the classification is a lot simpler, since the commonly used methods can only show the presence of amyloid in general rather than selectively colour individual types. For the limited differentiations possible between the various types it is necessary to block their staining reaction and compare the staining to an unblocked stained section. The possibilities are given in the chart below.
| Treatment | Amyloid affected |
|---|---|
| Autoclave at 120°C for 30 min. | AA no longer stains with congo red. |
| Autoclave at 120°C for 2 hours. | AL no longer stains with congo red. Pre-albumin related amyloid shows no change. |
| Potassium permanganate oxidation. | AA and β2-microglobulin amyloid no longer stain with congo red. |
| Alkaline guanidine for 1 minute. | AA no longer stains with congo red |
| Alkaline guanidine for 2 hours. | AL and systemic senile amyloid no longer stain with congo red. Familial amyloid protein retains congo red staining |
Immunohistochemistry is the most definitive method for classification of amyloid types by identification of their predominant protein. Amyloid fibrils may also be solubilized and identified in solution, although that is outside the scope of StainsFile.
The methods used for demonstrating amyloid are listed below. Of these, the DMAB nitrite test is a histochemical method for tryptophan using p-dimethylaminobenzaldehyde. The metallic impregnations are rarely used. By far, the most common method and something of a standard, if that word can be applied to amyloid staining, is to use the direct cotton dye, congo red, with the green birefringence it usually imparts.
The term direct cotton dye is used in the textile industry to refer to those dyes which can colour cotton without requiring a mordant, i.e. by direct dyeing. They are usually acid dyes. In the Colour Index their functional name is “direct + colour + number“. Some direct cotton dyes are suitable for staining amyloid, notably congo red (direct red 28) and sirius red F3B (direct red 80). Other direct cotton dyes, such as benzo scarlet 4BNS, sold under the proprietary name “amyloid red” (direct red 72), may also be successful. A reminder that any staining method for amyloid may be unsuccessful, and that applies to congo red and the other direct cotton dyes as well.
References
- Amyloid and Amyloidosis
http://en.wikipedia.org/wiki/Amyloid and http://en.wikipedia.org/wiki/Amyloidosis.
The charts were blended and reproduced under the Creative Commons licence.
Wikipedia - Amyloidosis
http://emedicine.medscape.com/article/335414-overview.
Medscape - Structure of amyloid
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2005.05025.x/full.
FEBS Journal - Carleton, H M, and Leach, E H, (1938).
Histological technique., Ed. 2.
Oxford University Press, London, England. - Cohen, A S, and Calkins, E, (1959).
Electron Microscopic Observations on a Fibrous Component in Amyloid of Diverse Origins
Nature, v. 183: p. 1202-1203. - Drury, R A, and Wallington, E A, (1967).
Carleton’s histological technique., Ed. 4.
Oxford University Press, London, England. - Elghetany, M.T. & Saleem, M.,
Methods for staining amyloid in tissues: a review.,
Stain Technology, July 1988, pages 201-212. - Francis, R J, (1973).
Histopathology: Selected Topics,
Editied by H C Cook.
Bailliere Tindall, London, England.
Citing:
Virchow, R (1860),
Cellular Pathology, p.371
London, England. - Gueft, B, and Ghidoni, J J, (1963).
The site of formation and ultrastructure of amyloid
American Journal of pathology, v. 43: p. 837. - Lendrum, A C, Slidders, W, and Fraser S, (1972).
Renal hyalin: A study of amyloidosis and diabetic fibrinous vasculosis with new staining methods,
Journal of Clinical Pathology, v. 25, P. 373 - Wolman, M. (1971).
Amyloid, its nature and molecular structure: comparison of a new toluidine blue polarized light method with traditional procedures.
Laboratory Investigation, v. 25: p. 104-110.